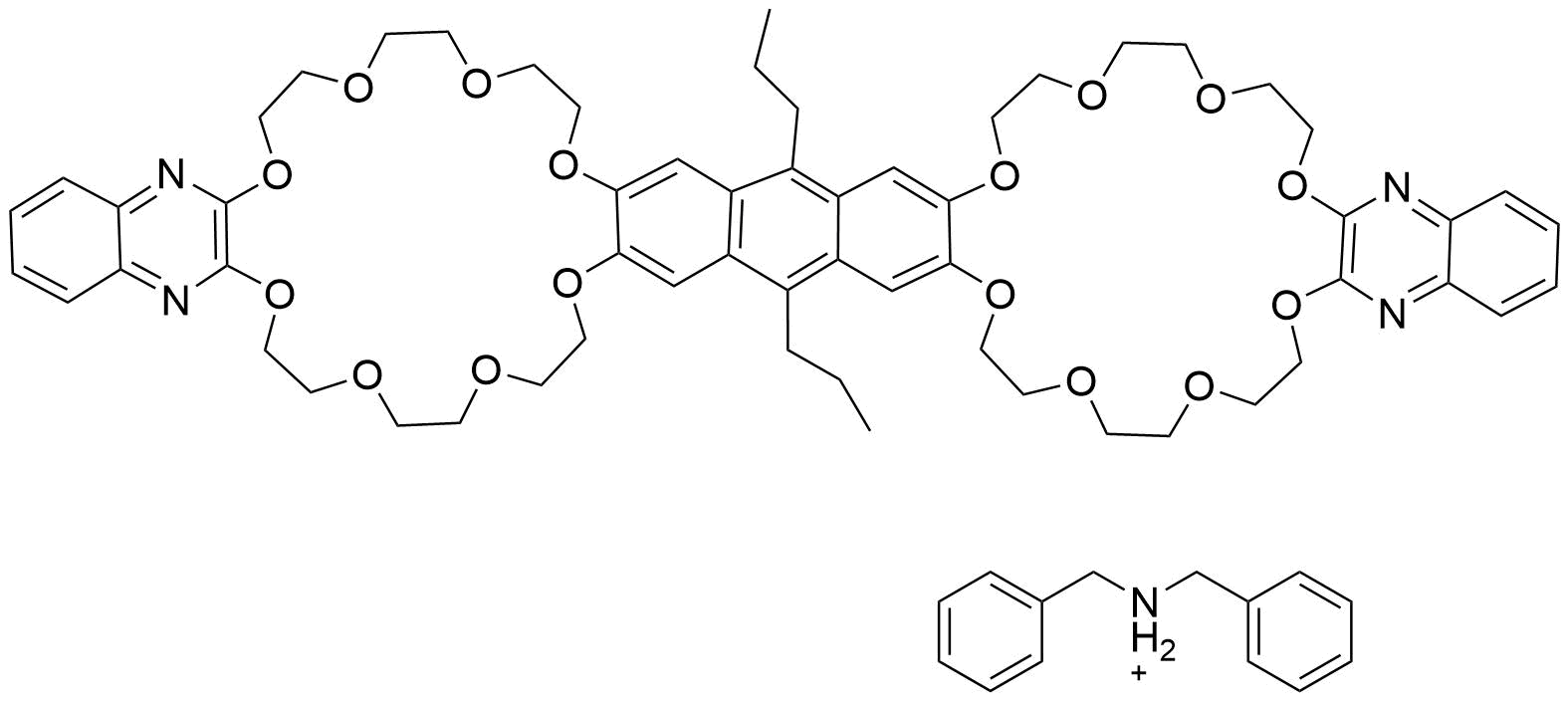
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 145.0 | ± 20.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -12.34 | ± 0.34 | -2.95 | ± 0.08 |
| ΔH | = | -75.2 | -17.97 | ||
| -TΔS | = | 62.9 | 15.03 | ||
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -211.0 | -50.4 | ||
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | acetonitrile | 67.0 % |
| chloroform | 33.0 % | |
| acetonitrile | ||
Please find here information about the dataset this interaction is part of.
| Citation: |
K. Nowosinski, L. K. S. von Krbek, N. L. Traulsen, C. A. Schalley, SupraBank 2024, Thermodynamic Analysis of Allosteric and Chelate Cooperativity in Di- and Trivalent Ammonium/Crown-Ether Pseudorotaxanes (dataset). https://doi.org/10.34804/supra.20210928210 |
| Link: | https://doi.org/10.34804/supra.20210928210 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
K. Nowosinski, L. K. S. von Krbek, N. L. Traulsen, C. A. Schalley, Org. Lett. 2015, 17, 5076–5079. |
| Link: | https://doi.org/10.1021/acs.orglett.5b02592 |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Dibenzylazanium (0.13793103448275862 M) and Bis[quinoxaline-2,3-diylbis[oxy(3,6-dioxaoctane-1,8-diyl)oxy]]-9,10-dipropylanthracene and on dibenzylammonium (0 — 0.27586206896551724 M).
Please sign in: customize the simulation by signing in to the SupraBank.