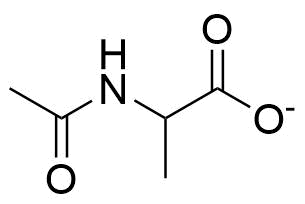
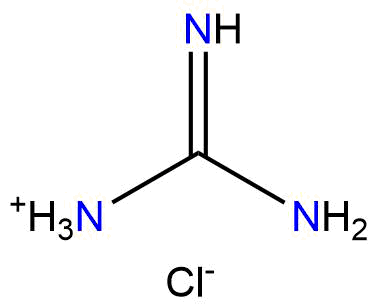
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka < | 10.0 | ± 68.0 | M-1 |
| Kd < | |||
| logKa < | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | > | -5.71 | ± NaN | -1.36 | ± NaN |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | DMSO-d6 | 60.0 % |
| water | 40.0 % | |
| water | ||
Please find here information about the dataset this interaction is part of.
| Citation: |
C. Schmuck, SupraBank 2024, Carboxylate Binding by 2-(Guanidiniocarbonyl)pyrrole Receptors in Aqueous Solvents: Improving the Binding Properties of Guanidinium Cations through Additional Hydrogen Bonds (dataset). https://doi.org/10.34804/supra.20210928209 |
| Link: | https://doi.org/10.34804/supra.20210928209 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
|
| Link: | https://doi.org/10.1002/(SICI)1521-3765(20000218)6:4%3C709::AID-CHEM709%3E3.0.CO;2-6 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of 2-Acetamidopropanoate (2.0 M) and Guanidinium Chloride (0 — 4.0 M).
Please sign in: customize the simulation by signing in to the SupraBank.