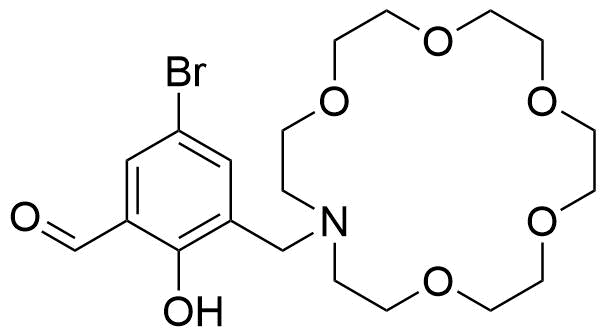
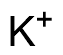Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.17⋅104 | ± 541.25 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -23.23 | ± 0.11 | -5.55 | ± 0.03 |
| ΔH | = | -41.5 | ± 0.2 | -9.92 | ± 0.05 |
| -TΔS | = | 18.3 | 4.37 | ||
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -61.4 | -14.7 | ||
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | methanol |
Please find here information about the dataset this interaction is part of.
| Citation: |
A. V. Bordunov, J. S. Bradshaw, V. N. Pastushok, X. X. Zhang, X. Kou, N. Kent Dalley, Z. Yang, P. B. Savage, R. M. Izatt, SupraBank 2024, Azacrown ethers containing oximic and Schiff base sidearms - potential heteronuclear metal ion receptors (dataset). https://doi.org/10.34804/supra.20210928115 |
| Link: | https://doi.org/10.34804/supra.20210928115 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
A. V. Bordunov, J. S. Bradshaw, V. N. Pastushok, X. X. Zhang, X. Kou, N. Kent Dalley, Z. Yang, P. B. Savage, R. M. Izatt, Tetrahedron 1997, 53, 17595–17606. |
| Link: | https://doi.org/10.1016/S0040-4020(97)10229-0 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of K+ (0.0017022754315693788 M) and 16-(2-Hydroxy-3-formyl-5-bromobenzyl)-1,4,7,10,13-pentaoxa-16-azacyclooctadecane (0 — 0.0034045508631387576 M).
Please sign in: customize the simulation by signing in to the SupraBank.