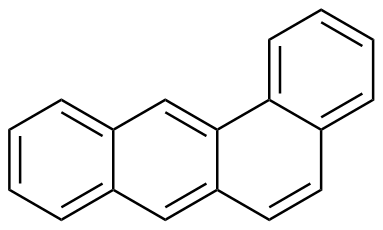
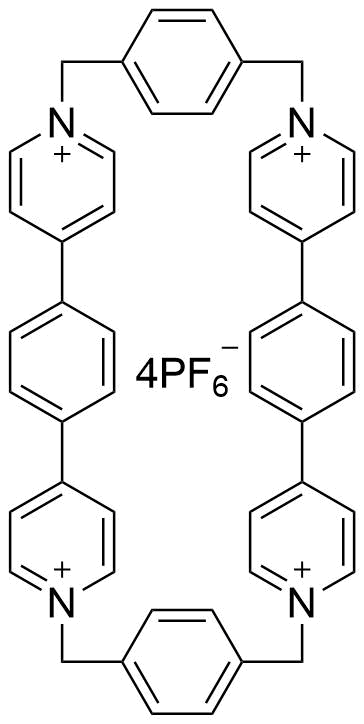
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 914.0 | ± 10.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -16.9 | ± 0.03 | -4.04 | ± 0.01 |
| ΔH | = | -24.23 | ± 0.12 | -5.79 | ± 0.03 |
| -TΔS | = | 7.36 | ± 0.12 | 1.76 | ± 0.03 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -24.7 | ± 0.4 | -5.9 | ± 0.1 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | MircoCal VP-ITC | ||
| VCell | = | 1800.0 𝜇L | |
| VSyringe | = | 350.0 𝜇L | |
| cmolecule | = | 15300.0 𝜇M syringe | |
| cpartner | = | 1020.0 𝜇M cell | |
| Ninjection | = | 25 | |
| Vinjection | = | 10.0 𝜇L | |
| Vinit | = | 10.0 𝜇L | |
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | acetonitrile |
Please find here information about the dataset this interaction is part of.
| Citation: |
J. C. Barnes, M. Juríček, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant, J. F. Stoddart, SupraBank 2024, ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger (dataset). https://doi.org/10.34804/supra.20210928187 |
| Link: | https://doi.org/10.34804/supra.20210928187 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
J. C. Barnes, M. Juríček, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant, et al., J. Am. Chem. Soc. 2012, 135, 183–192. |
| Link: | https://doi.org/10.1021/ja307360n |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Tetraphene (0.02188183807439825 M) and ExBox.4PF6 (0 — 0.0437636761487965 M).
Please sign in: customize the simulation by signing in to the SupraBank.