Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 3.00⋅105 | ± 3.00⋅104 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -31.26 | ± 0.25 | -7.47 | ± 0.06 |
| ΔH | = | -38.9 | ± 0.4 | -9.3 | ± 0.1 |
| -TΔS | = | 7.6 | ± 0.8 | 1.82 | ± 0.19 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -25.5 | ± 2.7 | -6.1 | ± 0.6 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Associative Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
| Cofactor: | syringe | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
O. A. Scherman, F. Biedermann, SupraBank 2026, Cucurbit[8]uril Mediated Donor–Acceptor Ternary Complexes: A Model System for Studying Charge-Transfer Interactions (dataset). https://doi.org/10.34804/supra.2021092867 |
| Link: | https://doi.org/10.34804/supra.2021092867 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
F. Biedermann, O. A. Scherman, J. Phys. Chem. B 2012, 116, 2842–2849. |
| Link: | https://doi.org/10.1021/jp2110067 |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
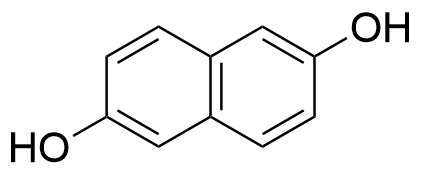
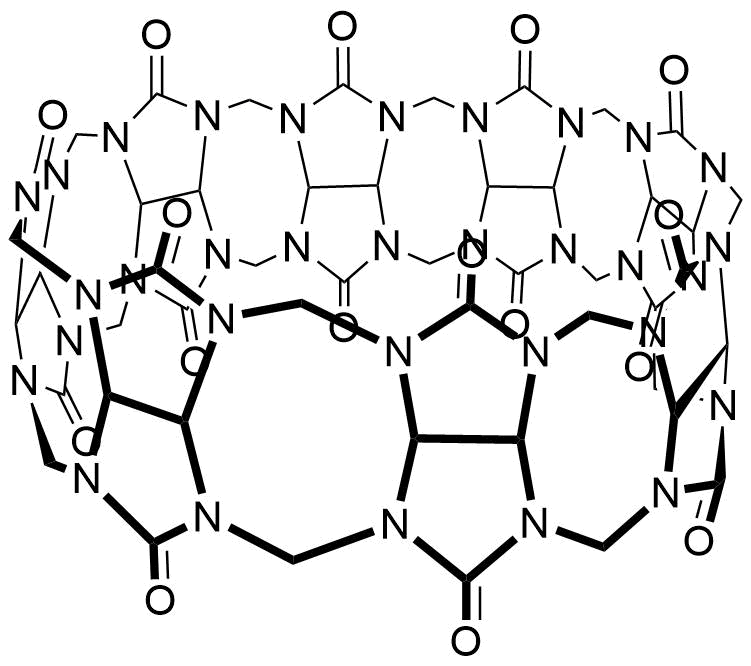
The plot depicts the binding isotherm simulation of a 1:1 interaction of 2,6-Dihydroxynaphthalene (6.666666666666667e-05 M) and CB8 (0 — 0.00013333333333333334 M).
Please sign in: customize the simulation by signing in to the SupraBank.