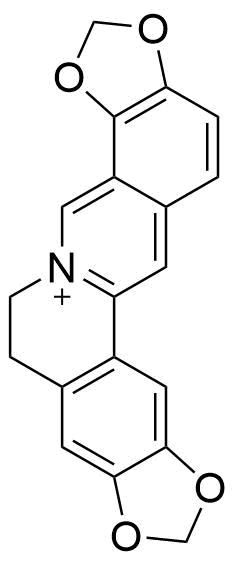
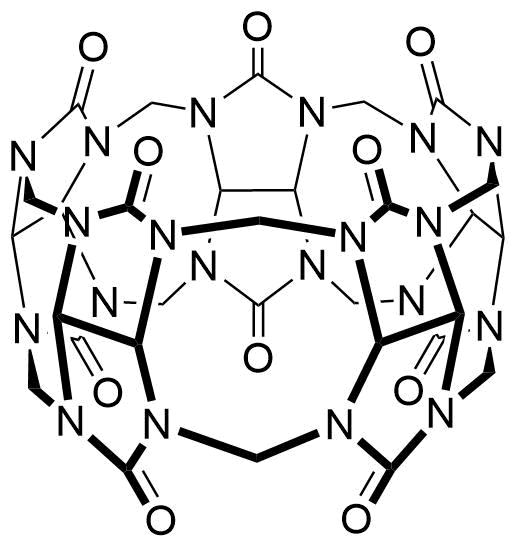
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.44⋅104 | ± | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -23.74 | ± 0.0 | -5.67 | ± 0.0 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 357.0 nm | ||
| 𝛌em | = | 521.0 nm | ||
| Ibound⁄Ifree | = | 8.0 | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 120 mM Britton–Robinson pH-2.0 |
| Solvents | water | |
| Additives | acetic acid | 40.0 mM |
| Phosphoric acid | 40.0 mM | |
| BORIC ACID | 40.0 mM | |
| Sodium hydroxide | ||
| Source of Concentration | real | |
| Total concentration | 120.0 mM | |
| pH | 2.0 |
Please find here information about the dataset this interaction is part of.
| Citation: |
C. Li, L. Du, W. Wu, A. Sheng, SupraBank 2026, Supramolecular interaction of cucurbit[n]urils and coptisine by spectrofluorimetry and its analytical application (dataset). https://doi.org/10.34804/supra.20210928313 |
| Link: | https://doi.org/10.34804/supra.20210928313 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
C.-F. Li, L.-M. Du, W.-Y. Wu, A.-Z. Sheng, Talanta 2010, 80, 1939–1944. |
| Link: | https://doi.org/10.1016/j.talanta.2009.10.049 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Coptisine (0.001388888888888889 M) and CB5 (0 — 0.002777777777777778 M).
Please sign in: customize the simulation by signing in to the SupraBank.