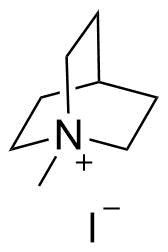
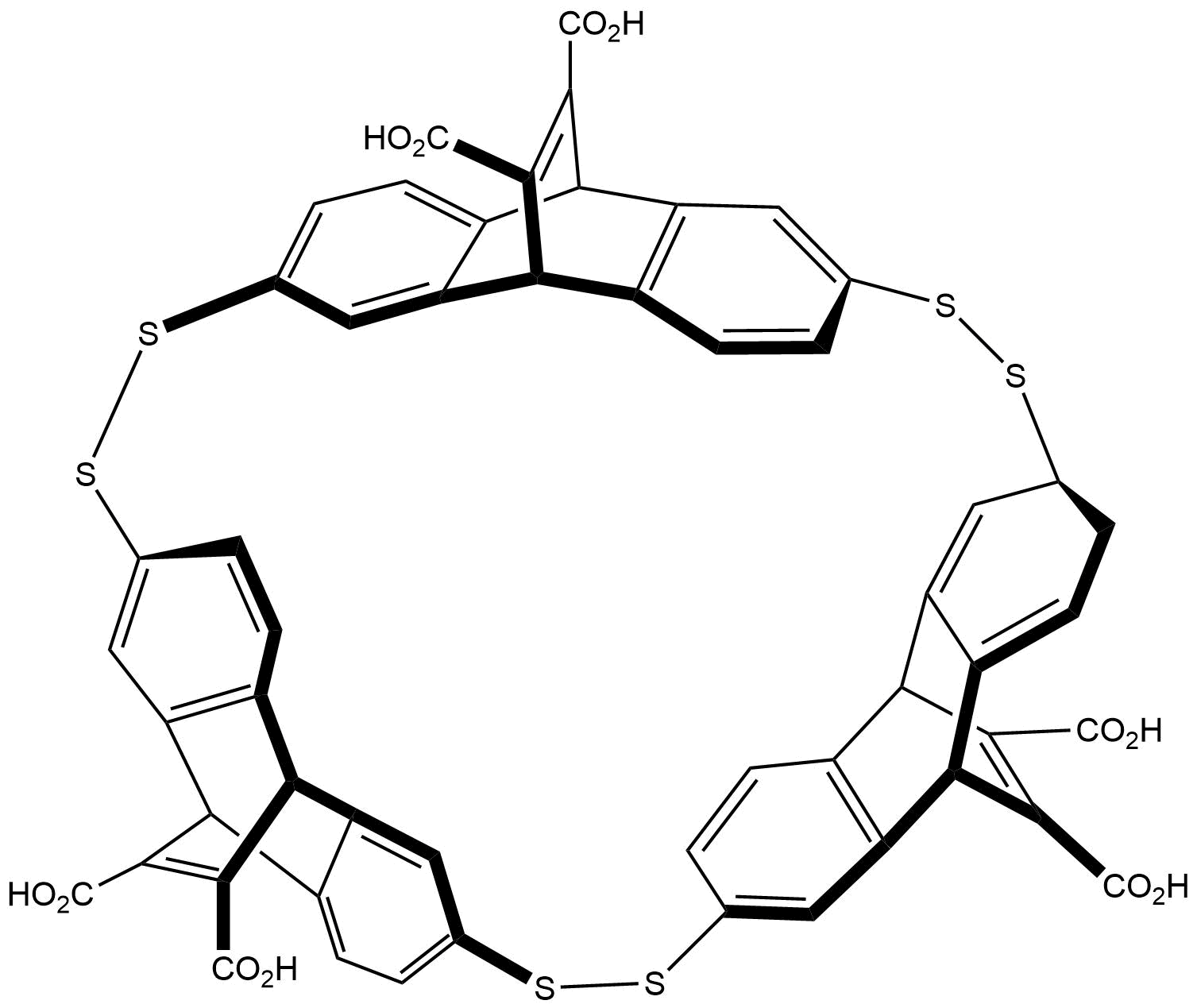
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 7.90⋅104 | ± 2000.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -27.96 | ± 0.06 | -6.68 | ± 0.01 |
| ΔH | = | -18.8 | ± 0.6 | -4.49 | ± 0.14 |
| -TΔS | = | -9.2 | ± 0.5 | -2.2 | ± 0.12 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | 30.9 | ± 1.7 | 7.4 | ± 0.4 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | MSC-ITC MicroCal Malvern Panalytical | ||
| Molecule: | syringe | ||
| Partner: | cell | ||
| Ninjection | = | 30 | |
| Vinjection | = | 10.0 𝜇L | |
| Vinit | = | 3.0 𝜇L | |
Detailed information about the solvation.
| Solvent System | Buffer System | 10 mM borate saline pH-9 |
| Solvents | water | 100.0 % |
| Additives | Sodium tetrabor... | 10.0 mM |
| sodium chloride | ||
| hydrochloric acid | ||
| Source of Concentration | ||
| Total concentration | 10.0 mM | |
| pH | 9.0 |
Please find here information about the dataset this interaction is part of.
| Citation: |
P. Corbett, J. Sanders, S. Otto, SupraBank 2026, Exploring the Relation between Amplification and Binding in Dynamic Combinatorial Libraries of Macrocyclic Synthetic Receptors in Water (dataset). https://doi.org/10.34804/supra.202109288 |
| Link: | https://doi.org/10.34804/supra.202109288 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
P. T. Corbett, J. K. M. Sanders, S. Otto, Chem. Eur. J. 2008, 14, 2153–2166. |
| Link: | https://doi.org/10.1002/chem.200701413 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of N-Methylquinuclidinium iodide (0.00025316455696202533 M) and Tri(9,10-dihydro-9,10-ethenoanthracene-11,12-dicarboxylic acid)-cyclophane (0 — 0.0005063291139240507 M).
Please sign in: customize the simulation by signing in to the SupraBank.