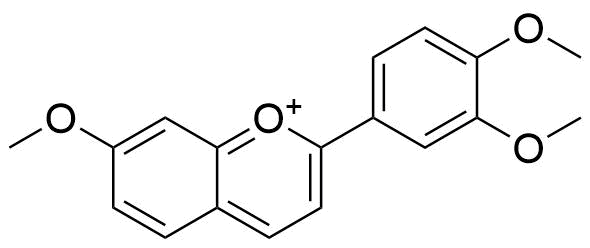
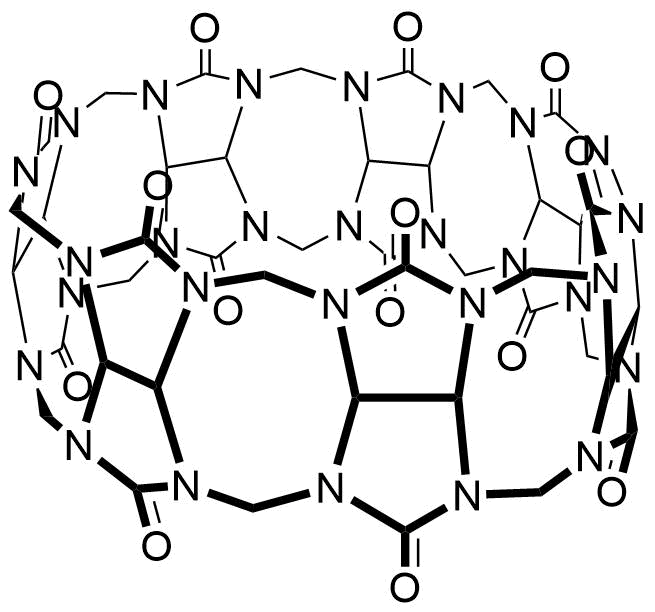
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.54⋅106 | ± 3.00⋅105 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 20.0 °C | ||
| kin= | 77000000.0 | ± 2000000.0 | M-1s-1 |
| kout= | 50.0 | ± 10.0 | s-1 |
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -34.73 | ± 0.48 | -8.3 | ± 0.11 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 470.0 nm | ||
| 𝛌em | = | 515.0 nm | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | water | |
| Additives | hydrochloric acid | 1.0 mM |
Please find here information about the dataset this interaction is part of.
| Citation: |
B. Held, H. Tang, P. Natarajan, C. P. da Silva, V. de Oliveira Silva, C. Bohne, F. H. Quina, SupraBank 2026, Cucurbit[7]uril inclusion complexation as a supramolecular strategy for color stabilization of anthocyanin model compounds (dataset). https://doi.org/10.34804/supra.2021092872 |
| Link: | https://doi.org/10.34804/supra.2021092872 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
B. Held, H. Tang, P. Natarajan, C. P. da Silva, V. de Oliveira Silva, C. Bohne, F. H. Quina, Photochem. Photobiol. Sci. 2016, 15, 752–757. |
| Link: | https://doi.org/10.1039/c6pp00060f |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Trimethoxyflavylium ion (1.2987012987012988e-05 M) and CB7 (0 — 2.5974025974025975e-05 M).
Please sign in: customize the simulation by signing in to the SupraBank.