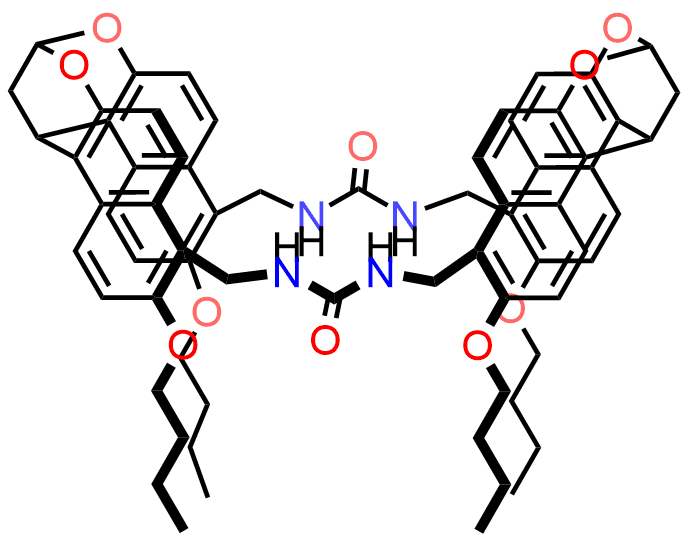
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.58⋅104 | ± 5900.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -23.97 | ± 0.97 | -5.73 | ± 0.23 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
| Nucleus | 2H | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | Chloroform-D |
Please find here information about the dataset this interaction is part of.
| Citation: |
G. Huang, A. Valkonen, K. Rissanen, W. Jiang, SupraBank 2026, endo-Functionalized molecular tubes: selective encapsulation of neutral molecules in non-polar media (dataset). https://doi.org/10.34804/supra.2021092840 |
| Link: | https://doi.org/10.34804/supra.2021092840 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
G. Huang, A. Valkonen, K. Rissanen, W. Jiang, Chem. Commun. 2016, 52, 9078–9081. |
| Link: | https://doi.org/10.1039/c6cc00349d |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Quinuclidine (0.0012658227848101266 M) and syn-Urea Naphthotube (0 — 0.002531645569620253 M).
Please sign in: customize the simulation by signing in to the SupraBank.