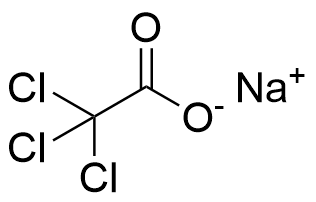
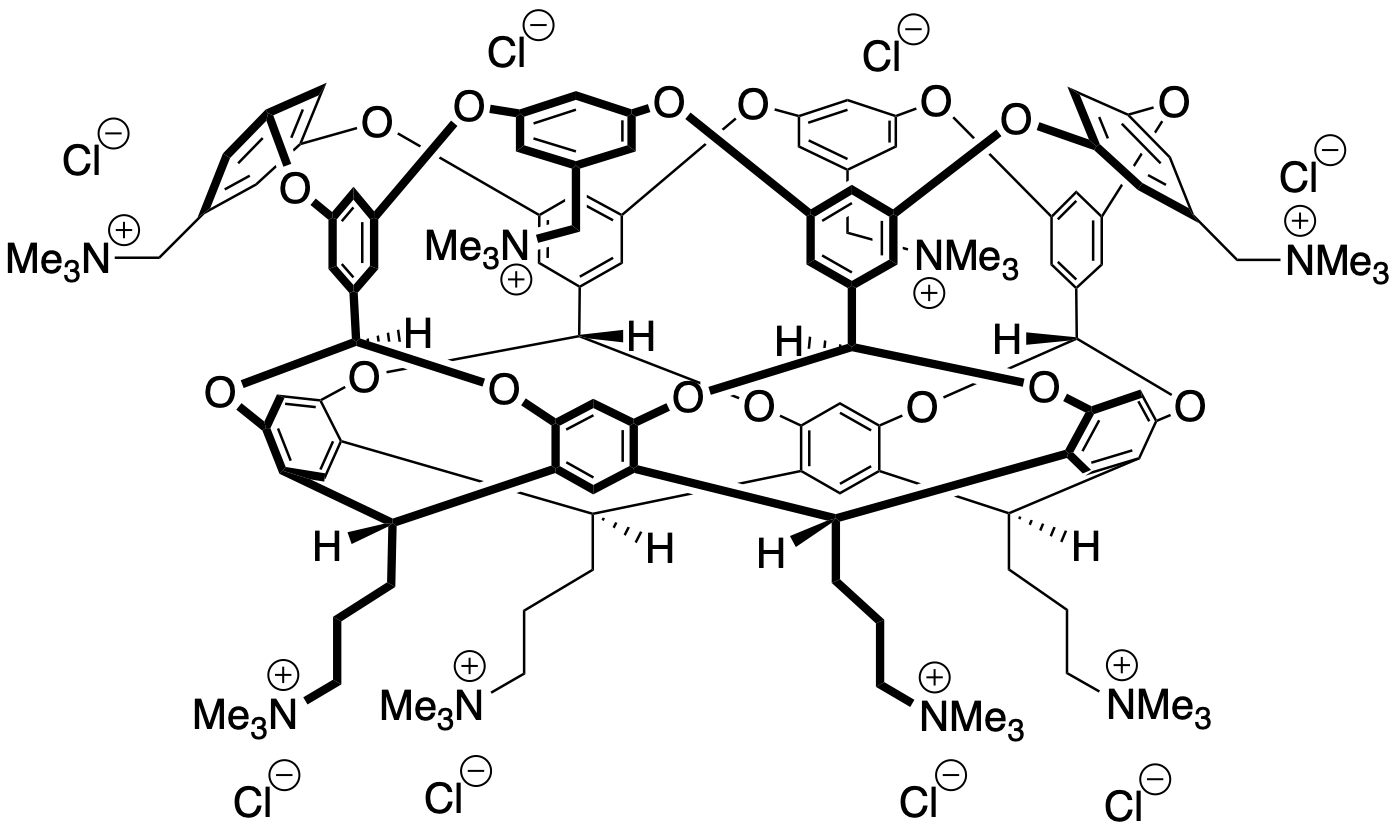
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 7.03⋅104 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -27.67 | -6.61 | ||
| ΔH | = | -24.18 | -5.78 | ||
| -TΔS | = | 3.47 | 0.83 | ||
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -11.6 | -2.8 | ||
- Comment
- Please check thermodynamic parameters.
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 10 mM phosphate pH-11.3 |
| Solvents | water | |
| Additives | Disodium phosph... | 8.0 mM |
| Trisodium phosp... | 2.0 mM | |
| Source of Concentration | ||
| Total concentration | 10.0 mM | |
| pH | 11.3 |
Please find here information about the dataset this interaction is part of.
| Citation: |
C. L. D. Gibb, B. Gibb, J. Jordan, A. Wishard, T. Pham, SupraBank 2026, Ion–Hydrocarbon and/or Ion–Ion Interactions: Direct and Reverse Hofmeister Effects in a Synthetic Host (dataset). https://doi.org/10.34804/supra.2021092870 |
| Link: | https://doi.org/10.34804/supra.2021092870 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
J. H. Jordan, C. L. D. Gibb, A. Wishard, T. Pham, B. C. Gibb, J. Am. Chem. Soc. 2018, 140, 4092–4099. |
| Link: | https://doi.org/10.1021/jacs.8b00196 |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Sodium trichloroacetate (0.0002844950213371266 M) and P1 (0 — 0.0005689900426742532 M).
Please sign in: customize the simulation by signing in to the SupraBank.