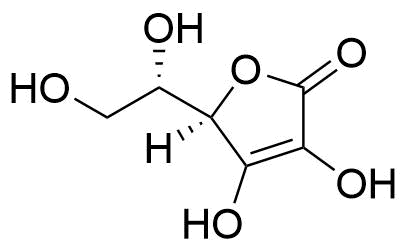
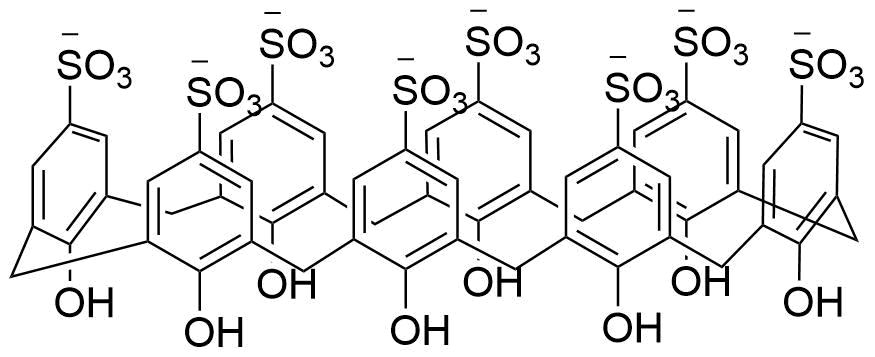
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 17.0 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -7.02 | -1.68 |
- Comment
- Buffer tablet for pH 4 used
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Absorbance | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture |
| Solvents | water |
| pH | 4.0 |
Please find here information about the dataset this interaction is part of.
| Citation: |
V. S. Kalyani, D. D. Malkhede, SupraBank 2026, p-Sulfonatocalix[8]arene and vitamin C complexation: assessment of photophysical, pKa and antioxidant property (dataset). https://doi.org/10.34804/supra.20210928252 |
| Link: | https://doi.org/10.34804/supra.20210928252 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
V. S. Kalyani, D. D. Malkhede, J Incl Phenom Macrocycl Chem 2017, 87, 179–189. |
| Link: | https://doi.org/10.1007/s10847-016-0689-x |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Ascorbic Acid (1.1764705882352942 M) and sCx8 (0 — 2.3529411764705883 M).
Please sign in: customize the simulation by signing in to the SupraBank.