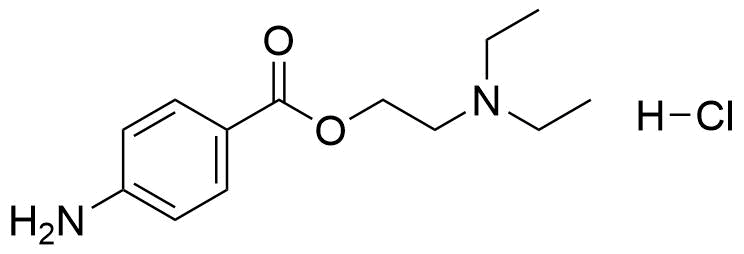
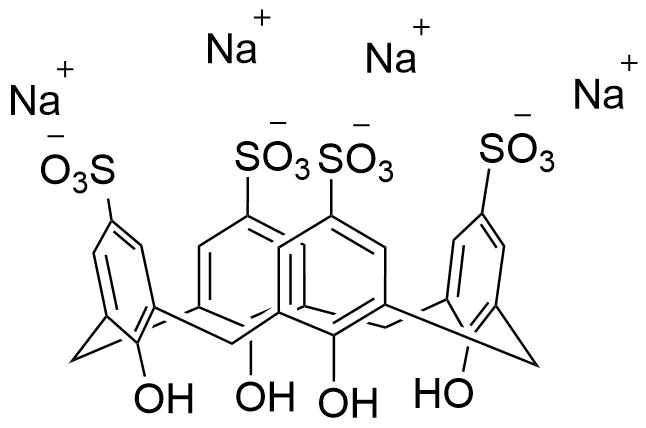
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 5090.0 | ± 80.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -21.16 | ± 0.04 | -5.06 | ± 0.01 |
| ΔH | = | -33.71 | ± 0.28 | -8.06 | ± 0.07 |
| -TΔS | = | 12.57 | ± 0.32 | 3.0 | ± 0.08 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -42.2 | ± 1.1 | -10.1 | ± 0.3 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 100 mM phosphate pH-7.2 |
| Solvents | water | |
| Additives | Disodium hydrog... | 66.6 mM |
| Sodium dihydrog... | 33.4 mM | |
| Source of Concentration | estimated | |
| Total concentration | 100.0 mM | |
| pH | 7.2 |
Please find here information about the dataset this interaction is part of.
| Citation: |
Q. Li, D. Guo, H. Qian, Y. Liu, SupraBank 2026, Complexation of p-Sulfonatocalixarenes with Local Anaesthetics Guests: Binding Structures, Stabilities, and Thermodynamic Origins (dataset). https://doi.org/10.34804/supra.202109283 |
| Link: | https://doi.org/10.34804/supra.202109283 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
Q. Li, D.-S. Guo, H. Qian, Y. Liu, Eur. J. Org. Chem. 2012, 2012, 3962–3971. |
| Link: | https://doi.org/10.1002/ejoc.201200515 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Procaine hydrochloride (0.003929273084479371 M) and sCx4 sodium salt (0 — 0.007858546168958742 M).
Please sign in: customize the simulation by signing in to the SupraBank.