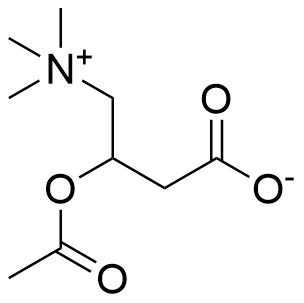
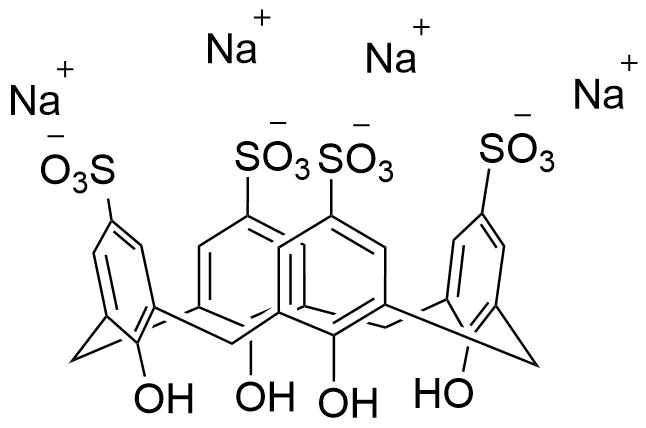
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 648.0 | ± 13.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -16.05 | ± 0.05 | -3.84 | ± 0.01 |
| ΔH | = | -25.76 | ± 0.25 | -6.16 | ± 0.06 |
| -TΔS | = | 9.71 | ± 0.04 | 2.32 | ± 0.01 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -32.6 | ± 0.1 | -7.8 | ± 0.0 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 100 mM phosphate pH-7.2 |
| Solvents | water | |
| Source of Concentration | estimated | |
| Total concentration | 100.0 mM | |
| pH | 7.2 |
Please find here information about the dataset this interaction is part of.
| Citation: |
D. Guo, Y. Liu, L. Wang, Y. Chen, SupraBank 2026, Thermodynamics of interactions between organic ammonium ions and sulfonatocalixarenes (dataset). https://doi.org/10.34804/supra.20210928383 |
| Link: | https://doi.org/10.34804/supra.20210928383 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
L.-H. Wang, D.-S. Guo, Y. Chen, Y. Liu, Thermochimica Acta 2006, 443, 132–135. |
| Link: | https://doi.org/10.1016/j.tca.2005.12.025 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Acetyl-D/L-Carnitine (0.030864197530864196 M) and sCx4 sodium salt (0 — 0.06172839506172839 M).
Please sign in: customize the simulation by signing in to the SupraBank.