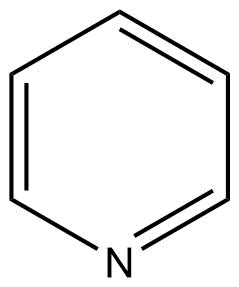
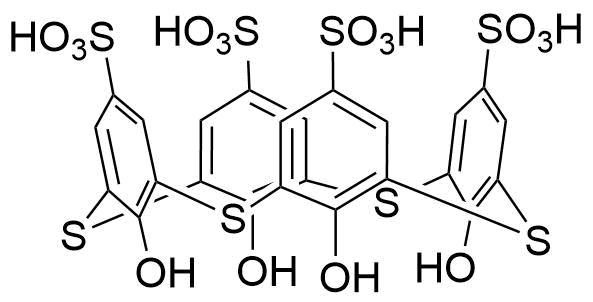
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 448.0 | ± 2.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -15.13 | ± 0.01 | -3.62 | ± 0.0 |
| ΔH | = | -19.95 | ± 0.15 | -4.77 | ± 0.04 |
| -TΔS | = | 4.83 | ± 0.15 | 1.15 | ± 0.04 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -16.2 | ± 0.5 | -3.9 | ± 0.1 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 100 mM phosphate pH-2.0 |
| Solvents | water | |
| Additives | Sodium dihydrog... | 100.0 mM |
| Phosphoric acid | ||
| Source of Concentration | ||
| Total concentration | 100.0 mM | |
| pH | 2.0 |
Please find here information about the dataset this interaction is part of.
| Citation: |
D. Guo, Y. Liu, F. Ding, E. Yang, Y. Chen, SupraBank 2026, Molecular Selective Binding of Pyridinium Guest Ions by Water-Soluble Calix[4]arenes (dataset). https://doi.org/10.34804/supra.20210928221 |
| Link: | https://doi.org/10.34804/supra.20210928221 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
Y. Liu, E.-C. Yang, Y. Chen, D.-S. Guo, F. Ding, Eur. J. Org. Chem. 2005, 2005, 4581–4588. |
| Link: | https://doi.org/10.1002/ejoc.200500354 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Pyridine (0.044642857142857144 M) and stCx4 (0 — 0.08928571428571429 M).
Please sign in: customize the simulation by signing in to the SupraBank.