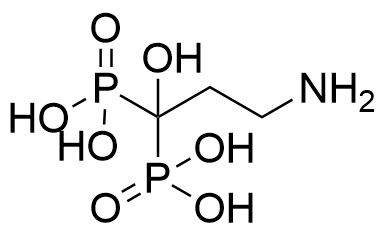
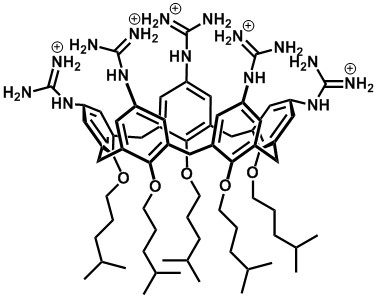
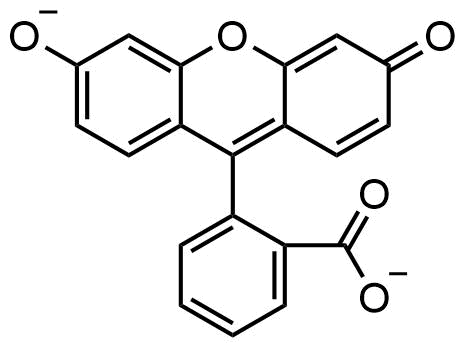
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 5.37⋅106 | ± 7.10⋅105 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -38.41 | ± 0.33 | -9.18 | ± 0.08 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | |||
| Assay Type: | Competitive Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 500.0 nm | ||
| 𝛌em | = | 514.0 nm | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 10 mM HEPES pH-7.4 |
| Solvents | water | 100.0 % |
| Additives | Hepes | 10.0 mM |
| Source of Concentration | ||
| Total concentration | 10.0 mM | |
| pH | 7.4 |
Please find here information about the dataset this interaction is part of.
| Citation: |
J. Gao, D. Guo, Z. Zheng, L. Shi, S. Wu, H. Sun, SupraBank 2026, Strong binding and fluorescence sensing of bisphosphonates by guanidinium-modified calix[5]arene (dataset). https://doi.org/10.34804/supra.20210928151 |
| Link: | https://doi.org/10.34804/supra.20210928151 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
J. Gao, Z. Zheng, L. Shi, S.-Q. Wu, H. Sun, D.-S. Guo, Beilstein J. Org. Chem. 2018, 14, 1840–1845. |
| Link: | https://doi.org/10.3762/bjoc.14.157%20 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of pamidronate (3.7243947858472997e-06 M) and gCx5-6C (0 — 7.448789571694599e-06 M).
Please sign in: customize the simulation by signing in to the SupraBank.