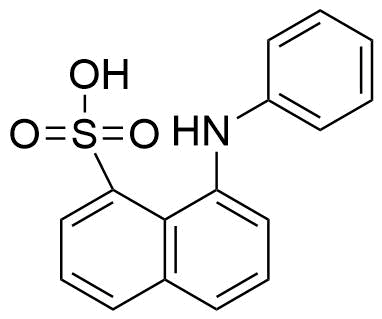
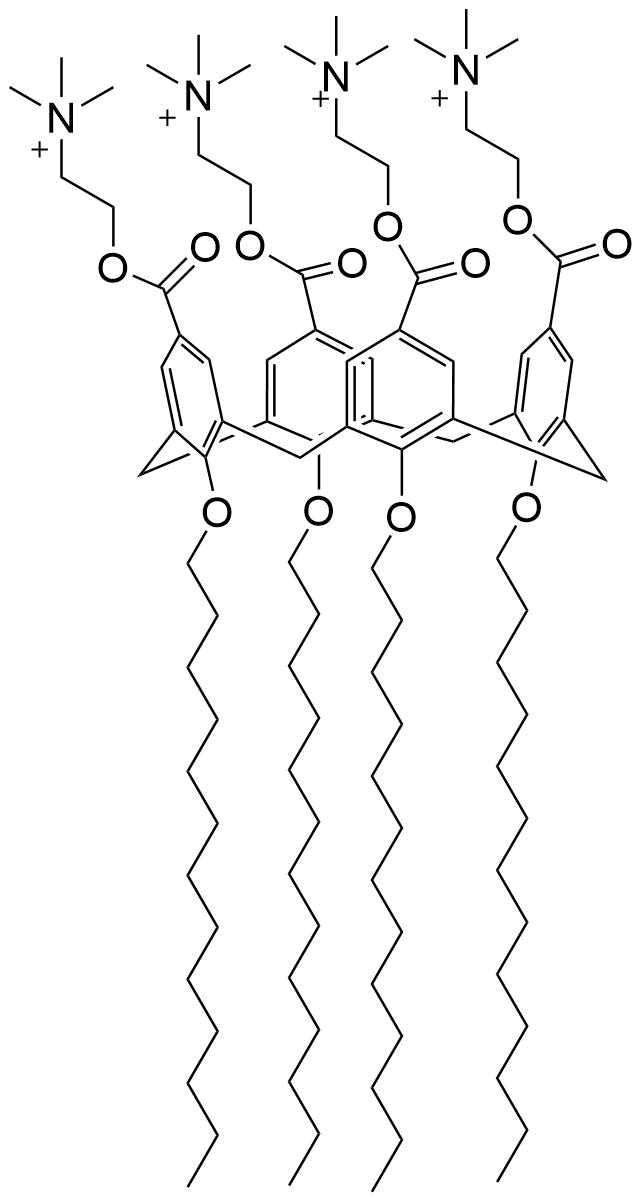
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 2.30⋅106 | ± 3.00⋅105 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -36.31 | ± 0.33 | -8.68 | ± 0.08 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 354.0 nm | ||
| 𝛌em | = | 465.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
| pH | 7.0 |
Please find here information about the dataset this interaction is part of.
| Citation: |
D. Guo, A. I. Lazar, Z. Xu, S. Peng, Y. Wang, J. Zhang, SupraBank 2026, Broad-Spectrum Tunable Photoluminescent Nanomaterials Constructed from a Modular Light-Harvesting Platform Based on Macrocyclic Amphiphiles (dataset). https://doi.org/10.34804/supra.2021092896 |
| Link: | https://doi.org/10.34804/supra.2021092896 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
Z. Xu, S. Peng, Y.-Y. Wang, J.-K. Zhang, A. I. Lazar, D.-S. Guo, Adv. Mater. 2016, 28, 7666–7671. |
| Link: | https://doi.org/10.1002/adma.201601719 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of 1,8-ANS (8.695652173913043e-06 M) and ChCx4-12C (0 — 1.7391304347826085e-05 M).
Please sign in: customize the simulation by signing in to the SupraBank.