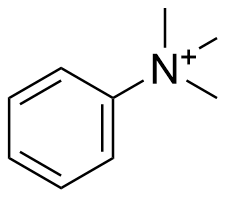
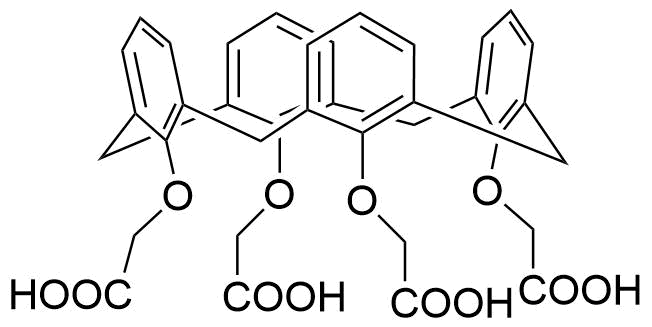
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 158.49 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -12.56 | -3.0 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
| Nucleus | 1H | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 100 mM deuterated phosphate pD-7.3 |
| Solvents | Deuterium Oxide | |
| Additives | Disodium deuter... | |
| Sodium dideuter... | ||
| Source of Concentration | estimated | |
| Total concentration | 100.0 mM | |
| pH | 7.3 |
Please find here information about the dataset this interaction is part of.
| Citation: |
G. Arena, D. Sciotto, A. Casnati, A. Contino, G. G. Lombardo, R. Ungaro, SupraBank 2026, ["Water-Soluble Calixarene Hosts that Specifically Recognize the Trimethylammonium Group or the Benzene Ring of Aromatic Ammonium Cations: A Combined1H NMR, Calorimetric, and Molecular Mechanics Investigation"] (dataset). https://doi.org/10.34804/supra.20210928340 |
| Link: | https://doi.org/10.34804/supra.20210928340 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
G. Arena, A. Casnati, A. Contino, G. G. Lombardo, D. Sciotto, R. Ungaro, Chemistry - A European Journal 1999, 5, 738–744. |
| Link: | https://doi.org/10.1002/(SICI)1521-3765(19990201)5:2%253C738::AID-CHEM738%253E3.0.CO;2-6 |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Phenyltrimethylammonium (0.12619092687235786 M) and Cx4-CH2COOH (0 — 0.2523818537447157 M).
Please sign in: customize the simulation by signing in to the SupraBank.