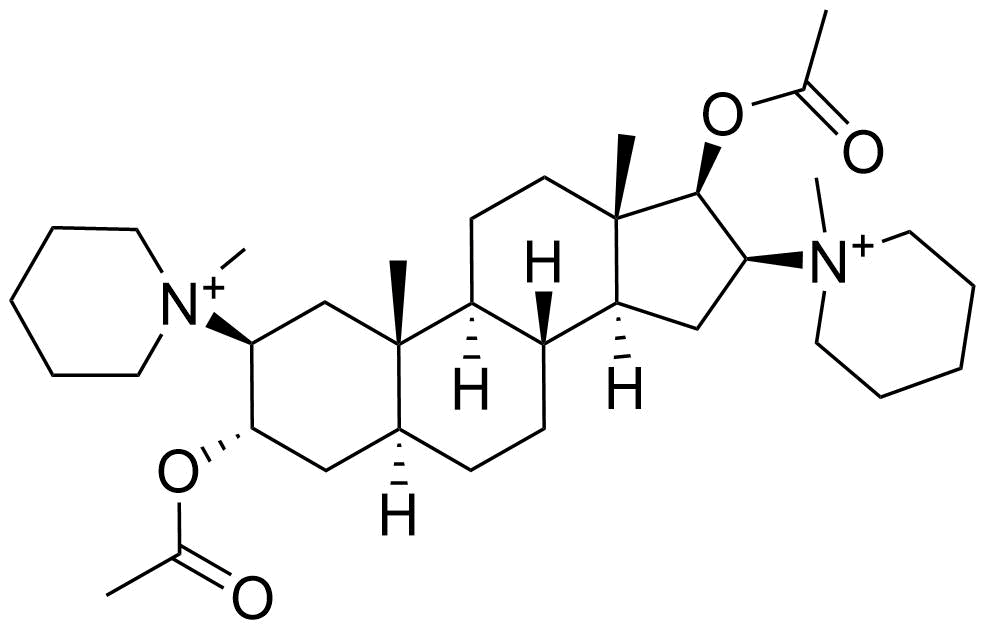
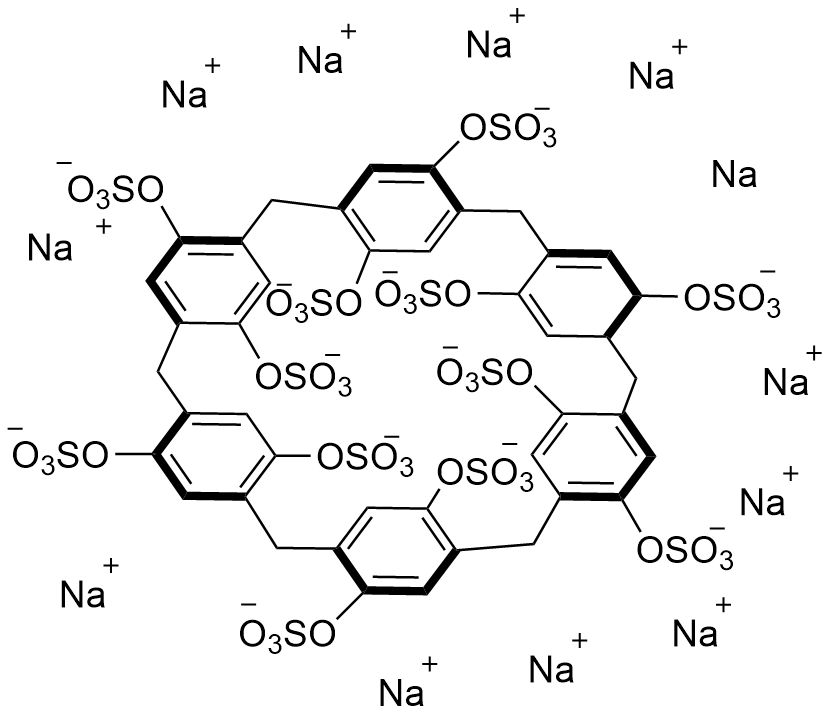
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 7.35⋅1010 | ± 1.23⋅1010 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -62.03 | ± 0.42 | -14.83 | ± 0.1 |
| ΔH | = | -69.04 | ± 0.9 | -16.5 | ± 0.22 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | ||
| Assay Type: | Competitive Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | syringe | ||
| Indicator: | syringe | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 20 mM phosphate pH-7.4 |
| Solvents | water | |
| Additives | Sodium dihydrog... | 20.0 mM |
| Source of Concentration | ||
| Total concentration | 20.0 mM | |
| pH | 7.4 |
Please find here information about the dataset this interaction is part of.
| Citation: |
W. Xue, P. Zavalij, L. Isaacs, SupraBank 2021, Pillar[ n ]MaxQ: A New High Affinity Host Family for Sequestration in Water (dataset). https://doi.org/10.34804/supra.2021092890 |
| Link: | https://doi.org/10.34804/supra.2021092890 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
W. Xue, P. Y. Zavalij, L. Isaacs, Angew. Chem. Int. Ed. 2020, 59, 13313–13319. |
| Link: | https://doi.org/10.1002/anie.202005902 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Pancuronium (2.7210884353741494e-10 M) and sP6A (0 — 5.442176870748299e-10 M).
Please sign in: customize the simulation by signing in to the SupraBank.