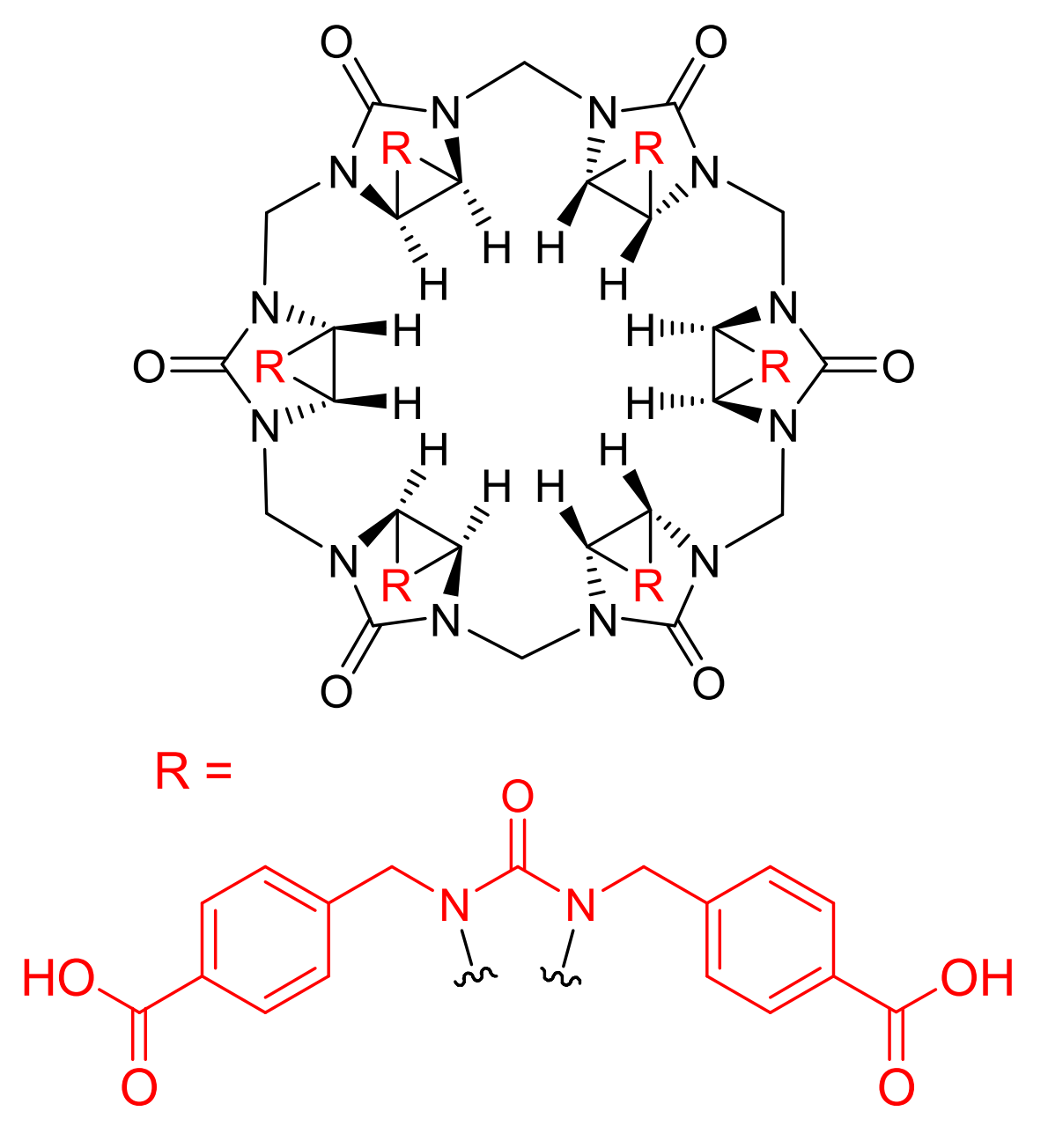
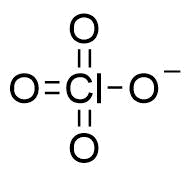Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 5.50⋅107 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 30.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -44.92 | -10.74 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | ||
| Assay Type: | Competitive Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | Deuterium Oxide | |
| Additives | Dipotassium deu... | 20.0 mM |
| Total concentration | 20.0 mM | |
| pH | 7.1 |
Please find here information about the dataset this interaction is part of.
| Citation: |
V. Havel, V. Sindelar, M. A. Yawer, SupraBank 2026, A Bambusuril Macrocycle that Binds Anions in Water with High Affinity and Selectivity (dataset). https://doi.org/10.34804/supra.2021092811 |
| Link: | https://doi.org/10.34804/supra.2021092811 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
M. A. Yawer, V. Havel, V. Sindelar, Angew. Chem. Int. Ed. 2014, 54, 276–279. |
| Link: | https://doi.org/10.1002/anie.201409895 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Perchlorate (3.6363636363636366e-07 M) and Dodeca(4-carboxybenzyl)bambus[6]uril (0 — 7.272727272727273e-07 M).
Please sign in: customize the simulation by signing in to the SupraBank.