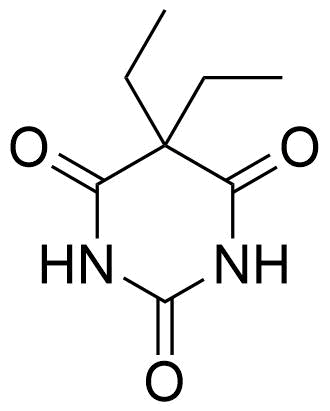
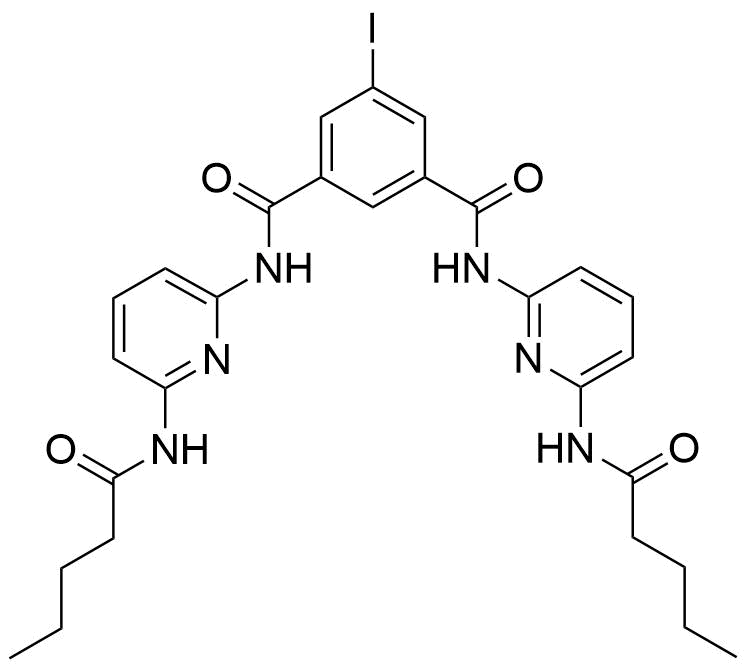
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 6.49⋅104 | ± 4800.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -27.47 | ± 0.18 | -6.57 | ± 0.04 |
- Comment
- NMR CIS measurement, further data avaiable based on NMR diffusion and ITC
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
| Nucleus | H-1 | ||
| = | 4.54 ppm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | chloroform |
Please find here information about the dataset this interaction is part of.
| Citation: |
C. Dethlefs, J. Eckelmann, H. Kobarg, T. Weyrich, S. Brammer, C. Näther, U. Lüning, SupraBank 2026, Determination of Binding Constants of Hydrogen-Bonded Complexes by ITC, NMR CIS, and NMR Diffusion Experiments (dataset). https://doi.org/10.34804/supra.20210928396 |
| Link: | https://doi.org/10.34804/supra.20210928396 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
C. Dethlefs, J. Eckelmann, H. Kobarg, T. Weyrich, S. Brammer, C. Näther, U. Lüning, Eur. J. Org. Chem. 2011, 2011, 2066–2074. |
| Link: | https://doi.org/10.1002/ejoc.201001684 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Barbital (0.0003081664098613251 M) and 5-Iodo-N,N'-bis[6-(pentanoylamino)pyrid-2-yl]isophthalamide (0 — 0.0006163328197226503 M).
Please sign in: customize the simulation by signing in to the SupraBank.