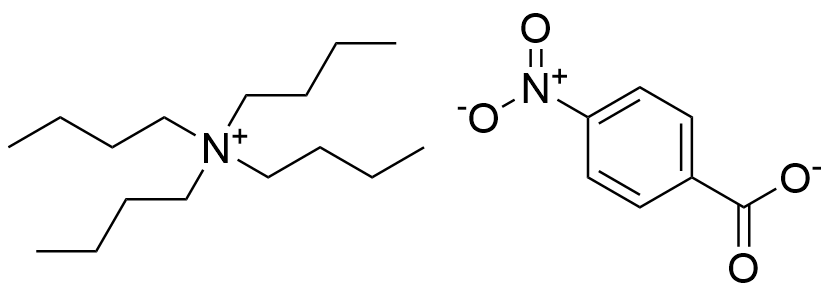
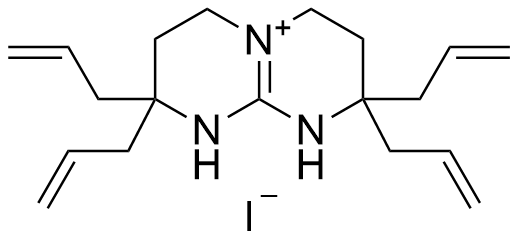
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.05⋅105 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 19.85 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -28.16 | -6.73 | ||
| ΔH | = | -22.26 | -5.32 | ||
| -TΔS | = | -5.9 | -1.41 | ||
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | 20.1 | 4.8 | ||
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Molecule: | syringe | ||
| Partner: | cell | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | acetonitrile |
Please find here information about the dataset this interaction is part of.
| Citation: |
V. D. Jadhav, F. P. Schmidtchen, SupraBank 2026, Surprises in the Design of Anion Receptors: Calorimetry Prevents False Reasoning (dataset). https://doi.org/10.34804/supra.2021092841 |
| Link: | https://doi.org/10.34804/supra.2021092841 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
V. D. Jadhav, F. P. Schmidtchen, Org. Lett. 2005, 7, 3311–3314. |
| Link: | https://doi.org/10.1021/ol051109k |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Tetrabutylammonium 4-nitrobenzoate (0.0001910858453160082 M) and tetraallyl-bicyclic guanidinium iodide (0 — 0.0003821716906320164 M).
Please sign in: customize the simulation by signing in to the SupraBank.