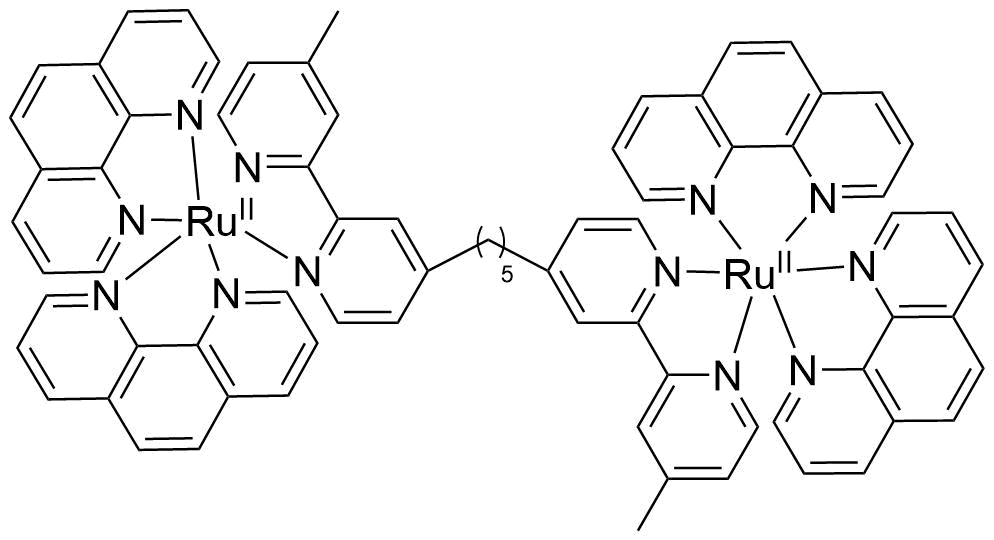
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka > | 1.90⋅109 | M-1 | |
| Kd > | |||
| logKa > | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | < | -52.96 | -12.66 |
- Comment
- emissions were recorded in the 570-650 nm range
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 435.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
L. Wallace, A. I. Day, F. R. Keene, J. G. Collins, M. J. Pisani, Y. Zhao, C. E. Woodward, SupraBank 2026, Cucurbit[10]uril binding of dinuclear platinum(II) and ruthenium(II) complexes: association/dissociation rates from seconds to hours (dataset). https://doi.org/10.34804/supra.20220629447 |
| Link: | https://doi.org/10.34804/supra.20220629447 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
M. J. Pisani, Y. Zhao, L. Wallace, C. E. Woodward, F. R. Keene, A. I. Day, J. G. Collins, Dalton Trans. 2010, 39, 2078. |
| Link: | https://doi.org/10.1039/B921172A |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of [{Ru(phen)2}2bb5] 4 (1.0526315789473684e-08 M) and CB10 (0 — 2.1052631578947368e-08 M).
Please sign in: customize the simulation by signing in to the SupraBank.