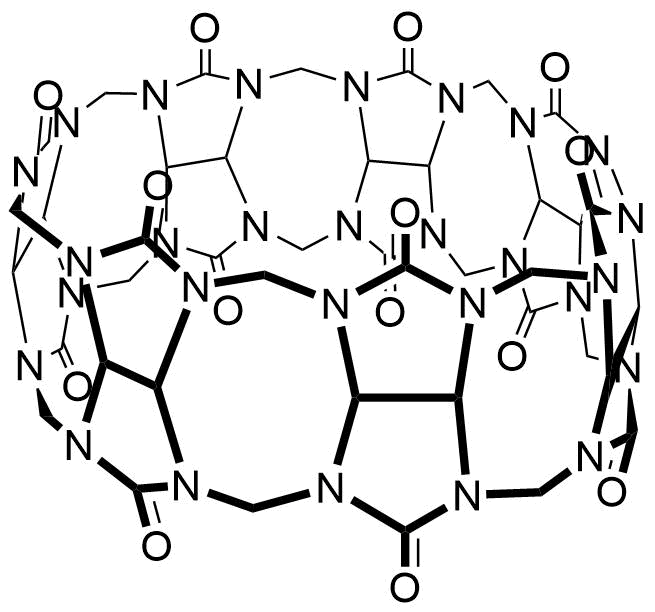
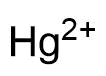Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1000.0 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -17.12 | -4.09 | ||
| ΔH | = | -91.0 | -21.75 | ||
| -TΔS | = | 74.0 | 17.69 | ||
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | -248.2 | -59.3 | ||
- Comment
- Binding constant,enthalphy and entropy are K2, ΔH2, ΔS2 as given in the paper respectively.
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | ||
| Assay Type: | Competitive Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | Nano ITC | ||
| VCell | = | 1000.0 𝜇L | |
| VSyringe | = | 250.0 𝜇L | |
| Molecule: | syringe | ||
| Partner: | cell | ||
| Indicator: | cell | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
Q. Wang, K. Wei, S. Huang, Q. Tang, Z. Tao, y. huang, SupraBank 2026, “Turn-Off” Supramolecular Fluorescence Array Sensor for Heavy Metal Ion Identification (dataset). https://doi.org/10.34804/supra.20221104463 |
| Link: | https://doi.org/10.34804/supra.20221104463 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
Q. Wang, K.-N. Wei, S.-Z. Huang, Q. Tang, Z. Tao, Y. Huang, ACS Omega 2021, 6, 31229–31235. |
| Link: | https://doi.org/10.1021/acsomega.1c04956 |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Hg2+ (0.02 M) and CB7 (0 — 0.04 M).
Please sign in: customize the simulation by signing in to the SupraBank.