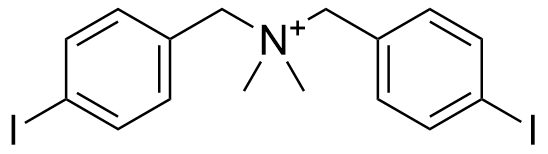
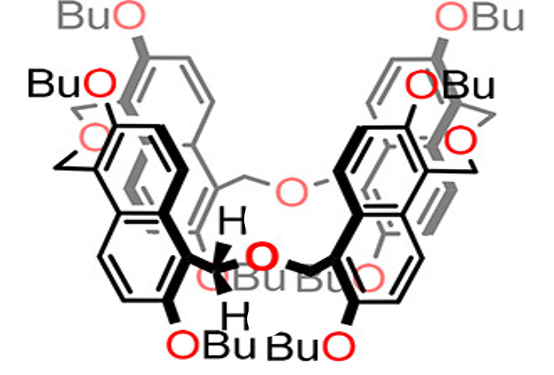
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.54⋅106 | ± 3.50⋅105 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -35.32 | ± 0.57 | -8.44 | ± 0.14 |
| ΔH | = | -26.4 | ± 2.64 | -6.31 | ± 0.63 |
| -TΔS | = | -8.9 | ± 0.89 | -2.13 | ± 0.21 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | 29.9 | ± 3.0 | 7.1 | ± 0.7 |
- Comment
- Counter ion is hexafluorophosphate anion.
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | Nano ITC LV | ||
| VCell | = | 190.0 𝜇L | |
| VSyringe | = | 50.0 𝜇L | |
| cmolecule | = | 1000.0 𝜇M syringe | |
| cpartner | = | 160.0 𝜇M cell | |
| Ninjection | = | 25 | |
| Vinjection | = | 1.96 𝜇L | |
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | 1,2-dichloroethane | 50.0 % |
| acetonitrile | 50.0 % | |
| Total concentration | 0.0 mM |
Please find here information about the dataset this interaction is part of.
| Citation: |
L. Yang, W. Jiang, F. Jia, D. Li, SupraBank 2026, Electronic Substituent Effects of Guests on the Conformational Network and Binding Behavior of Oxatub[4]arene (dataset). https://doi.org/10.34804/supra.20230308470 |
| Link: | https://doi.org/10.34804/supra.20230308470 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
F. Jia, L.-P. Yang, D.-H. Li, W. Jiang, J. Org. Chem. 2017, 82, 10444–10449. |
| Link: | https://doi.org/10.1021/acs.joc.7b01914 |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of N-(4-iodobenzyl)-1-(4-iodophenyl)-N,N-dimethylmethanaminium (1.2987012987012988e-05 M) and per-Butyl Oxatub[4]arene (0 — 2.5974025974025975e-05 M).
Please sign in: customize the simulation by signing in to the SupraBank.