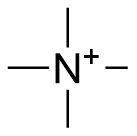
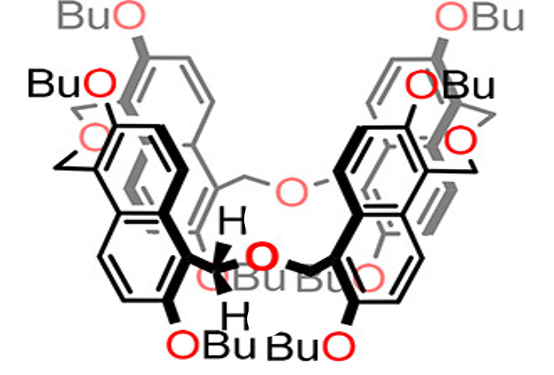
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 6388.0 | ± 600.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -21.72 | ± 0.23 | -5.19 | ± 0.05 |
| ΔH | = | -16.6 | ± 1.66 | -3.97 | ± 0.4 |
| -TΔS | = | -5.2 | ± 0.52 | -1.24 | ± 0.12 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | 17.4 | ± 1.7 | 4.2 | ± 0.4 |
- Comment
- Counter ion is hexafluorophosphate anion.
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | Nano ITC LV | ||
| VCell | = | 190.0 𝜇L | |
| VSyringe | = | 50.0 𝜇L | |
| cmolecule | = | 1000.0 𝜇M syringe | |
| cpartner | = | 160.0 𝜇M cell | |
| Ninjection | = | 25 | |
| Vinjection | = | 1.96 𝜇L | |
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | acetonitrile | 50.0 % |
| 1,2-dichloroethane | 50.0 % | |
Please find here information about the dataset this interaction is part of.
| Citation: |
W. Jiang, L. Yang, F. Jia, D. Li, H. Wang, SupraBank 2026, Oxatub[4]arene: a molecular “transformer” capable of hosting a wide range of organic cations (dataset). https://doi.org/10.34804/supra.20230630477 |
| Link: | https://doi.org/10.34804/supra.20230630477 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
F. Jia, H.-Y. Wang, D.-H. Li, L.-P. Yang, W. Jiang, Chem. Commun. 2016, 52, 5666–5669. |
| Link: | https://doi.org/10.1039/c6cc01052k |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Tetramethylammonium (0.0031308703819661866 M) and per-Butyl Oxatub[4]arene (0 — 0.006261740763932373 M).
Please sign in: customize the simulation by signing in to the SupraBank.