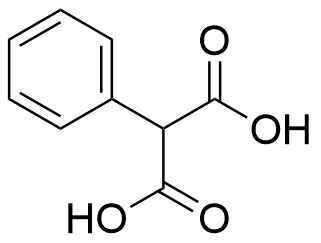
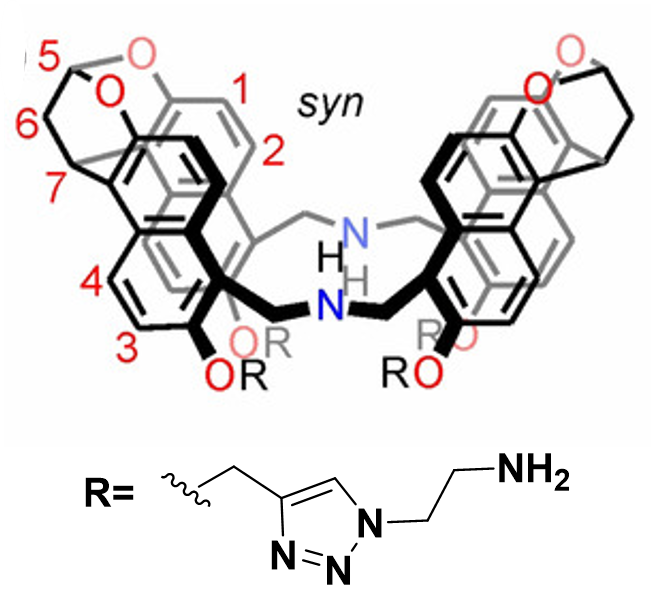
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 2.95⋅105 | ± 2.70⋅104 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -31.22 | ± 0.23 | -7.46 | ± 0.05 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
| Nucleus | H-1 | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 50mM acetate buffer deuterated |
| Solvents | Deuterium Oxide | |
| Source of Concentration | ||
| Total concentration | 50.0 mM | |
| pH | 5.43 |
Please find here information about the dataset this interaction is part of.
| Citation: |
X. Huang, X. Wang, M. Quan, H. Yao, H. Ke, W. Jiang, SupraBank 2023, Biomimetic Recognition and Optical Sensing of Carboxylic Acids in Water by Using a Buried Salt Bridge and the Hydrophobic Effect (dataset). https://doi.org/10.34804/supra.20230720484 |
| Link: | https://doi.org/10.34804/supra.20230720484 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
X. Huang, X. Wang, M. Quan, H. Yao, H. Ke, W. Jiang, Angew. Chem. Int. Ed. 2020, 60, 1929–1935. |
| Link: | https://doi.org/doi.org/10.1002/anie.202012467 |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Phenylmalonic acid (6.779661016949152e-05 M) and syn amine naphthotube (0 — 0.00013559322033898305 M).
Please sign in: customize the simulation by signing in to the SupraBank.