Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 2.20⋅104 | ± 3000.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -24.79 | ± 0.34 | -5.92 | ± 0.08 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Associative Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | MicroCal VP-ITC | ||
| VCell | = | 1400.0 𝜇L | |
| VSyringe | = | 350.0 𝜇L | |
| Molecule: | syringe | ||
| Partner: | cell | ||
| Cofactor: | cell | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
O. A. Scherman, F. Biedermann, U. Rauwald, S. Deroo, C. V. Robinson, SupraBank 2026, Correlating Solution Binding and ESI-MS Stabilities by Incorporating Solvation Effects in a Confined Cucurbit[8]uril System (dataset). https://doi.org/10.34804/supra.20231116505 |
| Link: | https://doi.org/10.34804/supra.20231116505 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
U. Rauwald, F. Biedermann, S. Deroo, C. V. Robinson, O. A. Scherman, J. Phys. Chem. B 2010, 114, 8606–8615. |
| Link: | https://doi.org/10.1021/jp102933h |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
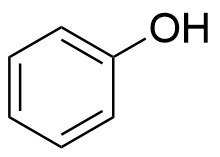
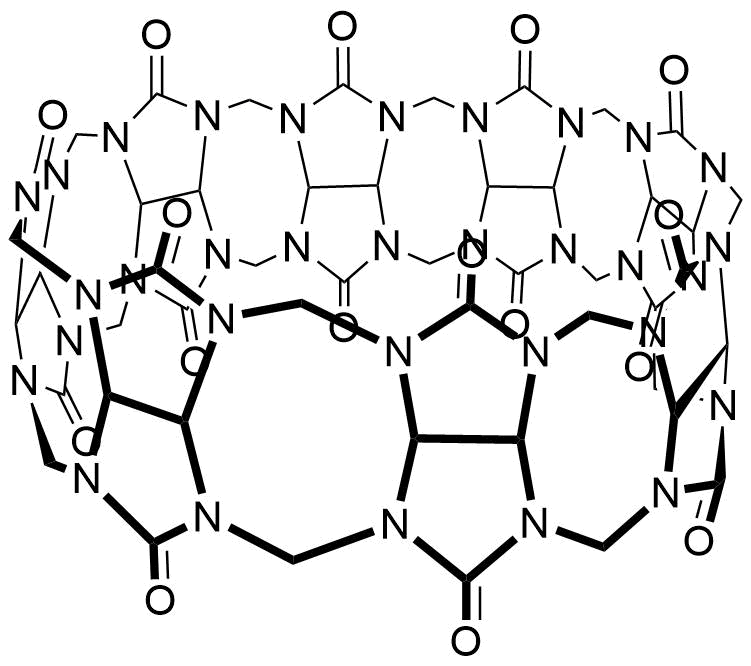
The plot depicts the binding isotherm simulation of a 1:1 interaction of Phenol (0.0009090909090909091 M) and CB8 (0 — 0.0018181818181818182 M).
Please sign in: customize the simulation by signing in to the SupraBank.