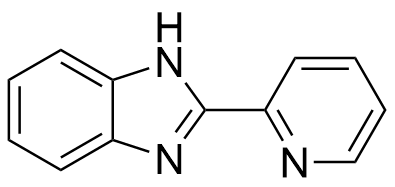
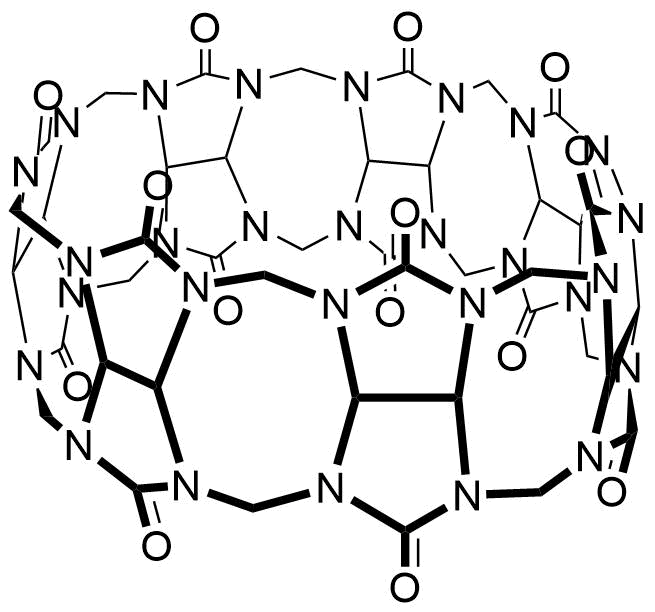
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1440.0 | ± 380.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -18.03 | ± 0.67 | -4.31 | ± 0.16 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 295.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
| pH | 9.4 |
Please find here information about the dataset this interaction is part of.
| Citation: |
S. A. Ahmed, D. Seth, SupraBank 2025, Thermodynamic analysis of binding of benzimidazole derivative with cucurbit[7]uril: A isothermal titration calorimetry study (dataset). https://doi.org/10.34804/supra.20240326535 |
| Link: | https://doi.org/10.34804/supra.20240326535 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
S. A. Ahmed, D. Seth, Journal of Molecular Liquids 2018, 254, 70–75. |
| Link: | https://doi.org/10.1016/j.molliq.2018.01.082%20 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of 2-PB (0.013888888888888888 M) and CB7 (0 — 0.027777777777777776 M).
Please sign in: customize the simulation by signing in to the SupraBank.