Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 9.00⋅104 | ± 6000.0 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -28.28 | ± 0.17 | -6.76 | ± 0.04 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | |||
| Assay Type: | Competitive Binding Assay | |||
| Technique: | Absorbance | |||
| 𝛌abs | = | 550.0 nm | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 25 mM sodium phosphate buffer, pH 7.4 |
| Source of Concentration | ||
| Total concentration | 25.0 mM | |
| pH | 7.4 |
Please find here information about the dataset this interaction is part of.
| Citation: |
L. Isaacs, P. Y. Zavalij, D. Ma, R. Glassenberg, S. Ghosh, SupraBank 2025, Acyclic cucurbituril congener binds to local anaesthetics (dataset). https://doi.org/10.34804/supra.20240423551 |
| Link: | https://doi.org/10.34804/supra.20240423551 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
D. Ma, R. Glassenberg, S. Ghosh, P. Y. Zavalij, L. Isaacs, Supramolecular Chemistry 2012, 24, 325–332. |
| Link: | https://doi.org/10.1080/10610278.2012.658394 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
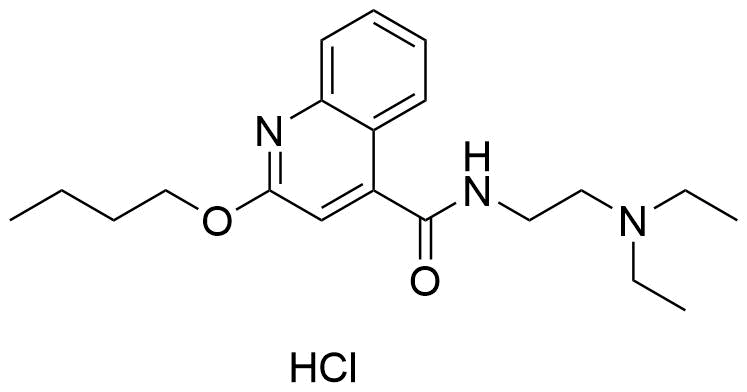
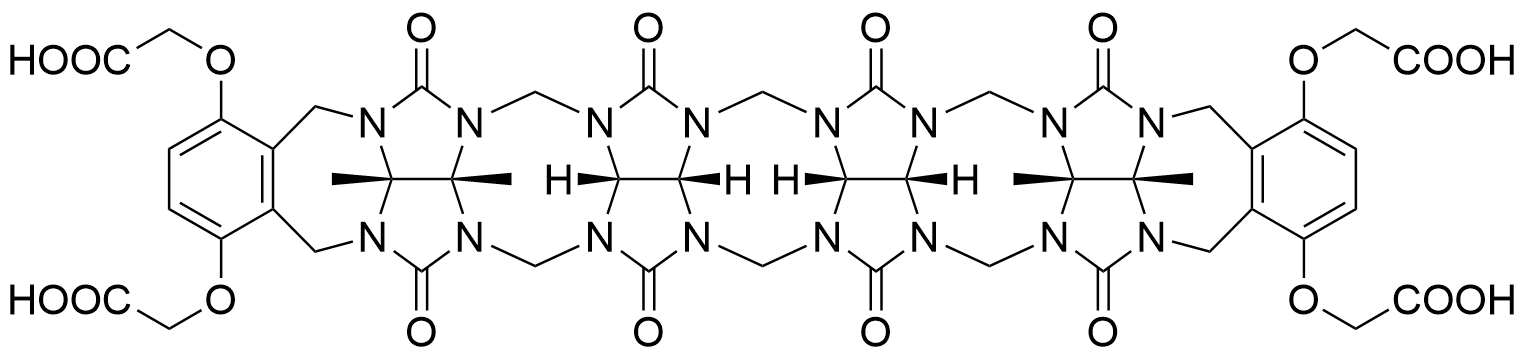
The plot depicts the binding isotherm simulation of a 1:1 interaction of Dibucaine hydrochloride (0.00022222222222222223 M) and CB5a (0 — 0.00044444444444444447 M).
Please sign in: customize the simulation by signing in to the SupraBank.