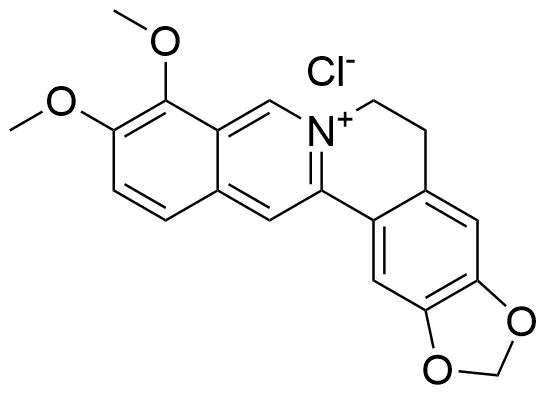
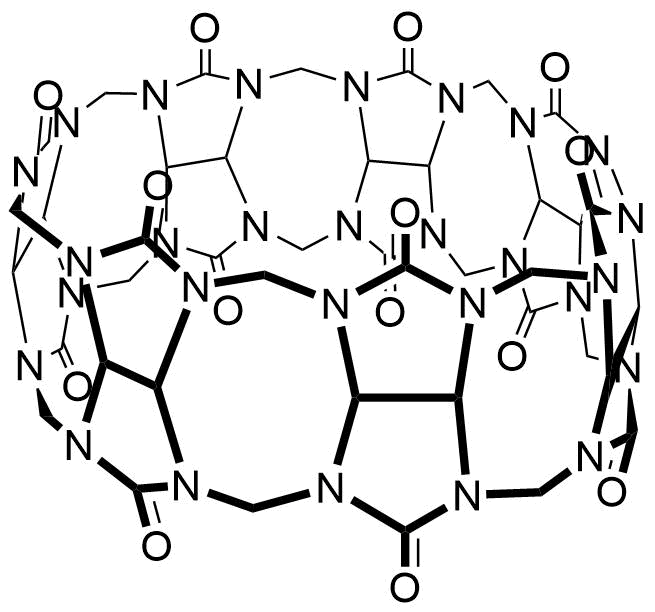
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 2.35⋅107 | ± 7.00⋅106 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| kin= | 19000000.0 | ± 1000000.0 | M-1s-1 |
| kout= | 0.810000004263158 | ± 0.08 | s-1 |
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -42.07 | ± 0.31 | -10.05 | ± 0.07 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 345.0 nm | ||
| 𝛌em | = | 500.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
Z. Miskolczy, L. Biczók, SupraBank 2026, Kinetics and Thermodynamics of Berberine Inclusion in Cucurbit[7]uril (dataset). https://doi.org/10.34804/supra.2021092813 |
| Link: | https://doi.org/10.34804/supra.2021092813 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
Z. Miskolczy, L. Biczók, J. Phys. Chem. B 2014, 118, 2499–2505. |
| Link: | https://doi.org/10.1021/jp500603g |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Berberine chloride (8.52631583434903e-07 M) and CB7 (0 — 1.705263166869806e-06 M).
Please sign in: customize the simulation by signing in to the SupraBank.