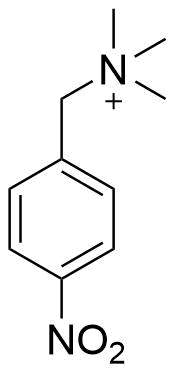
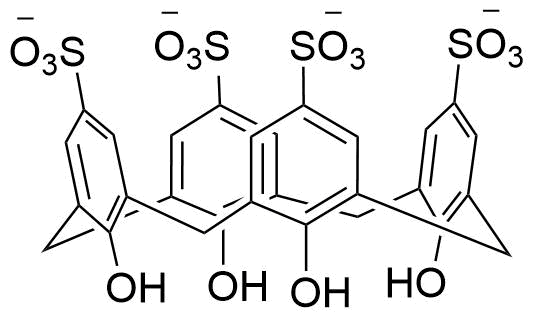
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1.58⋅104 | M-1 | |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -23.97 | -5.73 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Nuclear Magnetic Resonance | ||
| Nucleus | 1H | ||
Detailed information about the solvation.
| Solvent System | Buffer System | 100 mM deuterated phosphate pD-7.3 |
| Solvents | Deuterium Oxide | |
| Additives | Disodium deuter... | |
| Sodium dideuter... | ||
| Source of Concentration | estimated | |
| Total concentration | 100.0 mM | |
| pH | 7.3 |
Please find here information about the dataset this interaction is part of.
| Citation: |
G. Arena, S. Gentile, F. G. Gulino, D. Sciotto, C. Sgarlata, SupraBank 2026, Water-soluble pentasulfonatocalix[5]arene: selective recognition of ditopic trimethylammonium cations by a triple non-covalent interaction (dataset). https://doi.org/10.34804/supra.20210928270 |
| Link: | https://doi.org/10.34804/supra.20210928270 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
G. Arena, S. Gentile, F. G. Gulino, D. Sciotto, C. Sgarlata, Tetrahedron Letters 2004, 45, 7091–7094. |
| Link: | https://doi.org/10.1016/j.tetlet.2004.07.108 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of p-Nitrobenzyltrimethylammonium (0.0012619148422007038 M) and sCx4 (0 — 0.0025238296844014075 M).
Please sign in: customize the simulation by signing in to the SupraBank.