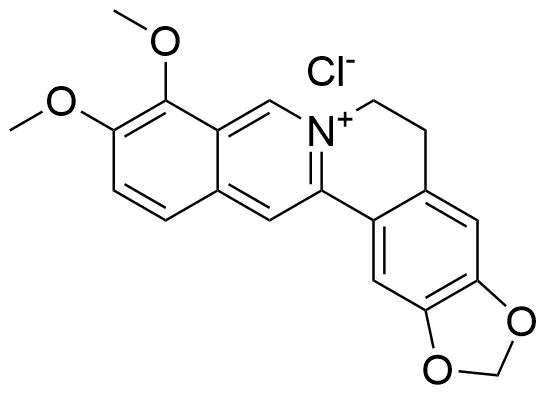
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 5.40⋅106 | ± 2.56⋅106 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 45.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -41.01 | ± 1.36 | -9.8 | ± 0.33 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | |||
| Assay Type: | Direct Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 440.0 nm | ||
| 𝛌em | = | 542.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
L. Grimm, SupraBank 2023, The Temperature-Dependence of Host-Guest Binding Thermodynamics: Experimental and Simulation Studies (dataset). https://doi.org/10.34804/supra.20220330425 |
| Link: | https://doi.org/10.34804/supra.20220330425 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
L. M. Grimm, J. Setiadi, B. Tkachenko, P. R. Schreiner, M. K. Gilson, F. Biedermann, Chem. Sci. 2023, DOI 10.1039/d3sc01975f. |
| Link: | https://doi.org/10.1039/D3SC01975F |
| Export: | BibTex | RIS | EndNote | |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of Berberine chloride (3.7037037037037037e-06 M) and CB7 (0 — 7.4074074074074075e-06 M).
Please sign in: customize the simulation by signing in to the SupraBank.