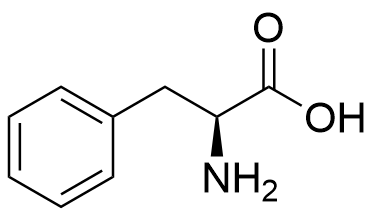
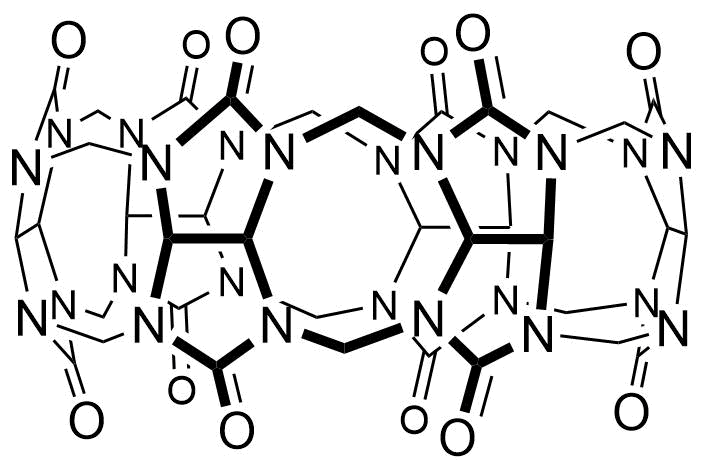
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 1445.0 | ± 33.3 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -18.04 | ± 0.06 | -4.31 | ± 0.01 |
| ΔH | = | -6.7 | ± 1.7 | -1.6 | ± 0.41 |
| -TΔS | = | -11.3 | ± 1.7 | -2.7 | ± 0.41 |
| J mol-1 K-1 | cal mol-1 K-1 | ||||
| ΔS | = | 37.9 | ± 5.7 | 9.1 | ± 1.4 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Direct | ||
| Assay Type: | Direct Binding Assay | ||
| Technique: | Isothermal Titration Calorimetry | ||
| Instrument: | ITC | ||
| Molecule: | syringe | ||
| Partner: | cell | ||
Detailed information about the solvation.
| Solvent System | Complex Mixture | |
| Solvents | water | 50.0 % |
| Formic acid | 50.0 % | |
Please find here information about the dataset this interaction is part of.
| Citation: |
H. Buschmann, E. Schollmeyer, L. Mutihac, SupraBank 2023, The formation of amino acid and dipeptide complexes with α-cyclodextrin and cucurbit[6]uril in aqueous solutions studied by titration calorimetry (dataset). https://doi.org/10.34804/supra.20231019500 |
| Link: | https://doi.org/10.34804/supra.20231019500 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
H.-J. Buschmann, E. Schollmeyer, L. Mutihac, Thermochimica Acta 2003, 399, 203–208. |
| Link: | https://doi.org/10.1016/S0040-6031(02)00462-8 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
The plot depicts the binding isotherm simulation of a 1:1 interaction of L-Phe (0.01384083044982699 M) and CB6 (0 — 0.02768166089965398 M).
Please sign in: customize the simulation by signing in to the SupraBank.