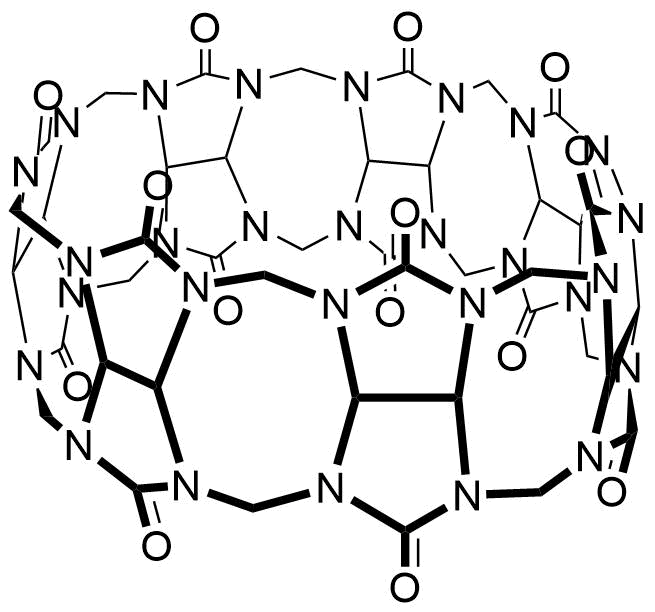
Binding Properties
| 𝜈 | Molecule 1 : 1 Host | ||
| Ka = | 9.70⋅108 | ± 1.94⋅108 | M-1 |
| Kd = | |||
| logKa = | |||
| T | 25.0 °C | ||
| Energy | kJ mol-1 | kcal mol-1 | |||
|---|---|---|---|---|---|
| ΔG | = | -51.3 | ± 0.5 | -12.26 | ± 0.12 |
These are the specifications of the determination of the experimental results.
| Detection Method: | Competitive | |||
| Assay Type: | Competitive Binding Assay | |||
| Technique: | Fluorescence | |||
| 𝛌ex | = | 421.0 nm | ||
| 𝛌em | = | 542.0 nm | ||
Detailed information about the solvation.
| Solvent System | Single Solvent |
| Solvent | water |
Please find here information about the dataset this interaction is part of.
| Citation: |
L. Grimm, SupraBank 2026, The Role of Packing, Dispersion, Electrostatics, and Solvation in High-Affinity Complexes of Cucurbit[n]urils with Uncharged Polar Guests (dataset). https://doi.org/10.34804/supra.20220323420 |
| Link: | https://doi.org/10.34804/supra.20220323420 |
| Export: | BibTex | RIS | EndNote |
Please find here information about the scholarly article describing the results derived from that data.
| Citation: |
L. M. Grimm, S. Spicher, B. Tkachenko, P. R. Schreiner, S. Grimme, F. Biedermann, Chemistry A European J 2022, 28, DOI 10.1002/chem.202200529. |
| Link: | https://doi.org/10.1002/chem.202200529 |
| Export: | BibTex | RIS | EndNote |
Binding Isotherm Simulations
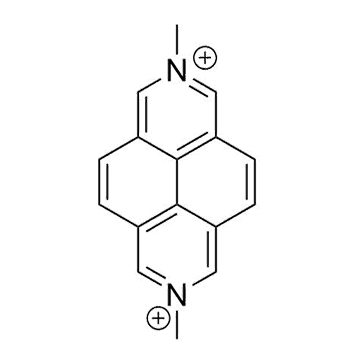
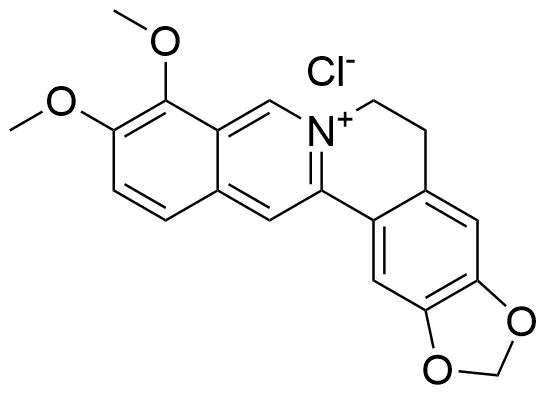
The plot depicts the binding isotherm simulation of a 1:1 interaction of MDAP (2.061855670103093e-08 M) and CB7 (0 — 4.123711340206186e-08 M).
Please sign in: customize the simulation by signing in to the SupraBank.